The Shorter a Wave's Wavelength the Greater Its Energy
The shorter a waves wavelength the greater its energy. 1 Why do shorter wavelengths of light.
What Is Difference Between Radiation And Wavelength Quora
Wavelength will become shorter.

. If the wavelength goes down then the frequency goes up. A high energy light will have a shorter wavelength than a low energy light. The equation E hv explains why shorter wavelengths have more energy than longer wavelengths.
The longer the wavelength of a wave the higher its energy. What happens when a frequency of a wave increases. In the Bohr model of the atom an element can only exist.
Click to see full answer. The shorter the wavelength of visible light the higher the frequency and the greater the energy of the photons. High-energy light has a short wavelength and a high frequency.
Hence the higher the frequency the shorter the wavelength and the higher the energy of the wave. Green light has a short wavelength and its bright because it carries much energy per unit area. Longer waves can carry more energy per unit area than shorter ones so theyre usually more powerful when exposed to the same amount of sunlight.
Similarly a photon wave with a longer wavelength will have a lower frequency and thus less energy. When calculating energy in the equation Ehv frequency v is. Given than the energy wavelength is directly.
The longer the wavelength of a wave the less it affects energy. Low-energy light has a long wavelength and a low frequency. This range is.
A shorter wavelength photon wave will have a higher frequency and consequently higher energy. The longer the wavelength the lower its energy. The energy of a wave is directly proportional to its frequency but inversely proportional to its wavelength.
The shorter the wavelength of a wave the lower its energy. E hn is the energy equation. Laser beam wavelengths can.
TRUE T or F. The shorter the wavelength the higher its energy. Red light blue light and purple light have even longer wavelengths and are also very bright.
The longer the wavelength the higher its energy. Peak energy is a term used to describe the energy produced by a pulsed laser where the time energy of the laser is delivered to a target over a short section of a pulse Lasers produce electromagnetic radiation light that typically propagate at a uniform wavelength. Spectral lines are produced when an electron makes a transition from one energy state to another.
Given than the energy wavelength is directly proportional to its frequency the frequency of shorter wavelengths typically measured in Hertz which is the number of waves in a specific time has a much lower energy than the longer wavelengths. As a result we can state that the. The shorter the wavelength of a wave the higher its energy.
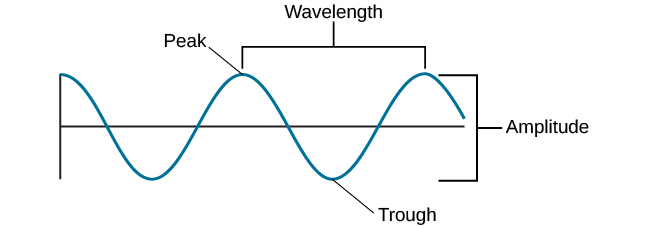
As a result the lower the energy the longer the wavelengths and the lower the frequency. Figure 61 The wave nature of light Light is a form of energy that travels with wavelike motion at a velocity of 300000000 meters per second. Similarly what happens to the energy and wavelength when you increase frequency.
Energy is connected to wavelength and frequency in the same way that wavelength and frequency are related to light. Figure 52 shows the wide range of electromagnetic radiation from AM radio waves with a wavelength of 10 4 m to gamma waves with a wavelength of 10-12 m. Frequency is always inversely related to the wavelength.
Also in this case a longer wavelength corresponds to lower wave energy whereas a shorter wavelength corresponds to higher wave energy. How is the wavelength of an EM wave related to its energy. The shorter the wavelength the lower its energy.
The greater the energy the shorter the wavelengths and higher the frequency. How does energy of the EM waves relate to their wavelength. In other words the greater the energy the larger the frequency and the shorter smaller the wavelength.
TRUE T or F. The wavelength is measured in nanometers. Shorter the wavelength the higher the frequency of the electromagnetic wave and greater is the energy of the waveform or the photon.
The energy associated with a wave is directly proportional to its frequency. Shorter wavelengths equate to higher frequency due to the c vlambda equation and frequency v is directly proportional to the energy of.

0 Response to "The Shorter a Wave's Wavelength the Greater Its Energy"
Post a Comment